

The Ret tyrosine kinase is a signaling receptor for the glial-derived neurotrophic factor (GDNF) family of ligands that are dynamically expressed in the peripheral tissues. The rapid adapting (RA) low threshold mechanosensory neurons were recently shown to derive from the early Ret-receptor expressing neurons. In the absence of Runx1, the afferents from these neurons move from innervating the more ventral lamina II to the most dorsal lamina I in the dorsal spinal cord. Non-peptidergic nociceptive sensory neurons require skin-derived NGF/TrkA signaling to induce/maintain the expression of Runx1 transcription factor, which then regulates the lamina-specific targeting in the spinal cord. The signals regulating layer-specific innervations into the spinal cord from three different classes of sensory neurons are summarized in Figure 1A. Considerably, increasing evidence suggests that both laminar-specific termination and topographic mapping depend on gene expressions induced by periphery-derived retrograde signals. The central axons of these sensory neurons have two important tasks: terminate onto distinct laminae/layers in the developing spinal cord according to their sensory modalities (functional classes), and project topographically based on their peripheral targets’ locations thereby forming a somatotopic map of the body surface. Sensory neurons in the mammalian somatosensory system have a pseudo-unipolar axon that stems from the cell body and bifurcates to form two main branches: one innervating a peripheral target and the other projecting into the spinal cord or the brainstem. Retrograde regulation of axon projections: target-derived signals received by peripheral axons control the projections of central axons in the somatosensory system We will review the studies related to retrograde specification of neuronal connections and circuit properties, highlight recent findings, and discuss the advantages afforded by the target-dependent mechanisms. Readers are referred to previously published excellent reviews for details on intracellular mechanisms of retrograde neurotrophin signaling, local neurotrophin signaling in axon outgrowth, and on neurotrophin-mediated rapid modulation of synaptic transmission and plasticity. Moreover, we will not discuss the roles of neurotrophins as neuronal survival factors or as synaptic transmission modulators. Other cell surface proteins that act as “recognition molecules” to guide the local interactions between growth cones and their targets are not subjects of this review.
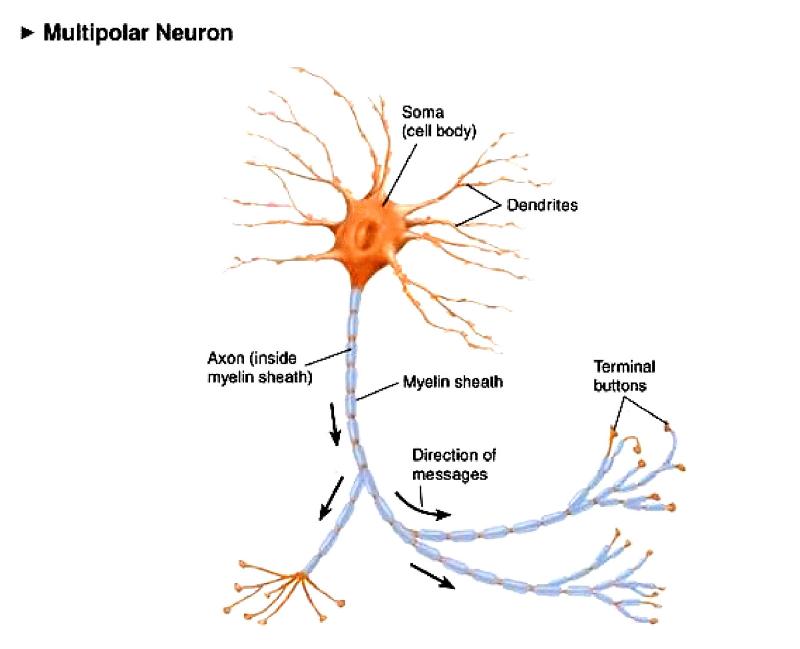
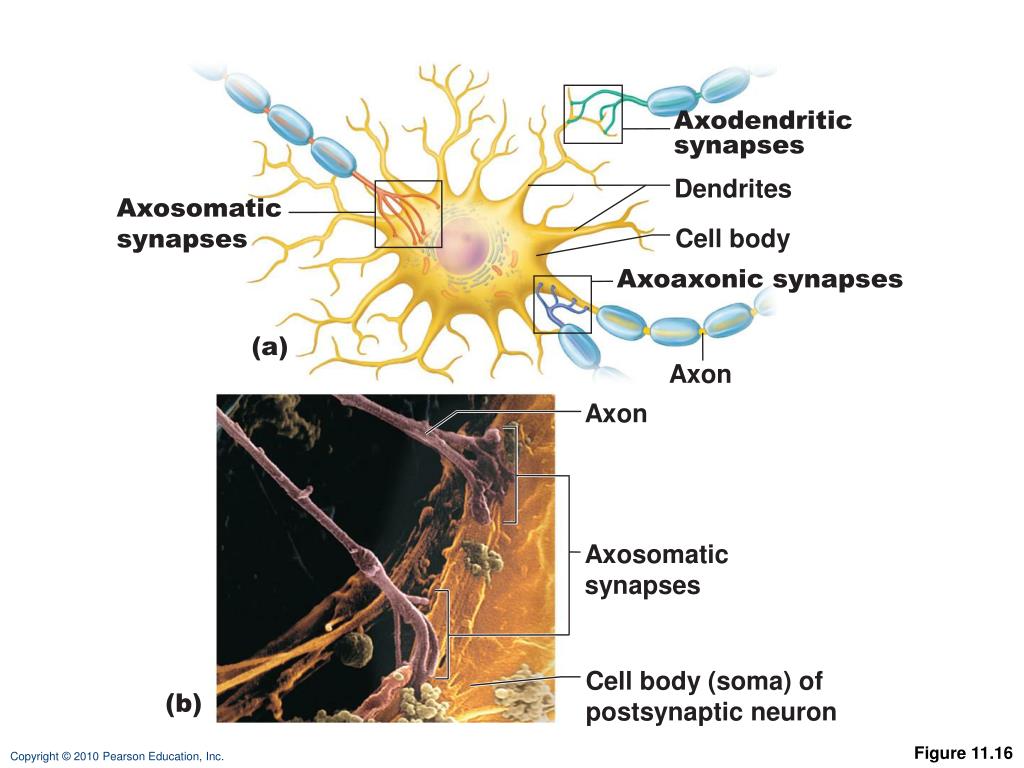
We will focus on neurotrophins and those growth factors that have the potentials to elicit long-range retrograde signaling from axon terminal to neuronal soma. Since the primary functions of axons are to deliver/forward information from the soma to their synaptic targets, this direction is generally called anterograde, and therefore the signals received by axons are considered as “retrograde”. Interestingly, in many types of neurons, target-derived signals can induce subsequent molecular and cellular changes, which are essential for the late steps of assembly and maturation of the relevant neural circuits. At the same time, the pre-specified programs also include the expressions of specific receptors for factors released by the neurons’ axonal targets (intermediary or final targets). These programs determine the early steps of neural circuit assembly including directed neuronal migrations, the initial axon pathfinding toward the target region, and the initiation of dendritic growth. The pre-specified programs control the transcriptional profiles of neural progenitor cells and early postmitotic neurons, which reflect the intrinsic cell fate differences and are independent of target contact. Genetically encoded programs can be further divided into pre-specified and target-dependent mechanisms. The assembly of complex yet specific neural circuits is controlled by both genetic programs and experience-dependent changes, i.e.


 0 kommentar(er)
0 kommentar(er)
